Abstract

Background/objective Andexanet alfa (AA) is a novel reversal agent for life threatening bleeding associated with oral Xa inhibitors, but there is uncertainty regarding the benefit compared to 4-factor prothrombin complex concentrate (4F-PCC). We investigated the clinical outcomes and cost differences between these two reversal agents in life threatening intracranial hemorrhage (ICH).
Methods We retrospectively reviewed patients at our institution who received anticoagulation reversal for life-threatening ICH with either AA or 4F-PCC between 9/1/2013 - 4/20/2020 and had an anti-Xa level greater than 0.6 IU/L. The primary outcome was 30-day mortality after administration of either 4F-PCC or AA. Secondary outcomes included progression of ICH based on imaging, ICU length of stay, hospital length of stay, disposition at discharge, functionality at discharged, and financial cost. Safety outcomes evaluated included thrombosis after administration of reversal agent.
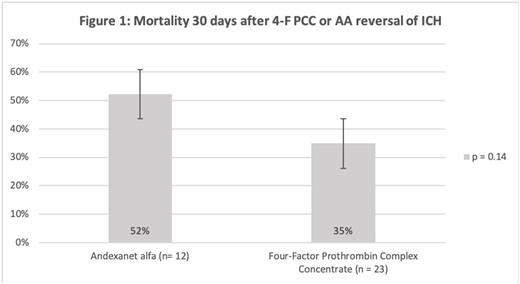
Results 89 patients were included in this study. There were no differences in 30-day mortality (p = 0.14, Figure 1), radiographic resolution of ICH (p = 0.93), objective volume change in ICH(p = 0.18), progression of ICH (p = 0.74), hospital and ICU length of stay (p=0.66, 0.50), discharge disposition (p=0.40), or thrombosis after administration of reversal agent (p=0.17) between the two groups. Modified Rankin at discharge was slightly higher for the AA group (p=0.02), while cost was 2.8 times higher for AA.
Conclusion Given the lack of statistically significant differences in clinical outcomes and the high cost of AA, the results validate the use of 4F-PCCs as an alternative to AA, based on similar efficacy and safety. Multi-center prospective studies are needed to further evaluate novel anticoagulation reversal agents.
Disclosures
Zumberg:Veralox Therapeutics: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.